
P4 Science – Heat & Temperature
You can download this Resource in PDF format to print or keep for future reference. Once you’ve submitted your request, it’ll be delivered straight to your email inbox.
Why Heat & Temperature Matter in Science and Everyday Life
Heat is a key topic in the Primary 4 Science syllabus that lays the foundation for understanding more complex concepts in Primary 5 topic, ‘Water Cycle & Changes in States’, such as melting, boiling, evaporation, condensation and freezing.
Many students confuse temperature with heat energy, or struggle to explain how heat flows and why materials expand or crack. This guide simplifies the concepts and includes structured templates for open-ended questions.
In this guide, your child will:
- learn to differentiate heat and temperature
- understand effects of heat gain or loss in terms of expansion and contraction
- understand how uneven expansion and contraction causes cracks and master the technique to explain how it happened
- learn more about real-life examples
What is Heat? What is Temperature?
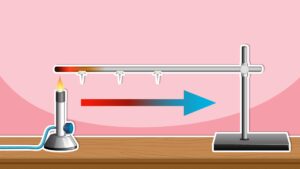
- Heat is a form of energy that flows from a hotter object/region (higher temperature) to a colder object/region (lower temperature) until they reach the same temperature.
- Heat can also flow within the same object from the hotter to colder region.
- Temperature is a measurement of how hot or cold something is.
- The amount of heat energy depends on volume and temperature.
For example:

- Given the same temperature, a bathtub filled with hot water (50°C) contains greater volume of water than a small cup of warm water (50°C).
- Therefore, the bathtub of water contains more heat energy.
Effects of Heat Gain: Change in Volume (Expansion/Contraction)
Key concepts to remember:
- When an object gains heat, it expands (increases in volume).
- when an object loses heat, it contracts (decreases in volume)
Uneven expansion and contraction can cause objects to crack when:
- One surface expands faster than another
- One surface contracts while another surface expands
- This difference causes stress, which leads to cracks in materials like glass or concrete.
Real-Life examples that your child can relate to
Example: Paul submerged a thick glass jar into a basin of ice and poured boiling water into it as shown below. He noticed that the jar started to crack. Explain his observation clearly.
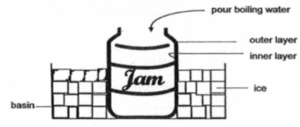
Step 1: Property (heat conductivity) of material – glass
– Glass is a poor conductor of heat.
Step 2: Effects of heat gain & heat loss
– The inner layer of glass gained heat from the boiling water and expanded.
– The outer layer of glass lost heat to the ice cubes and contracted.
Step 3: Link to outcome
Hence, the uneven expansion and contraction of the glass jar caused the jar to crack.
Example: Why did the glass cup crack when boiling water was poured into it?
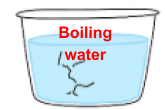
Step 1: Property (heat conductivity) of material – glass
Glass is a poor conductor of heat.
Step 2: Effects of heat gain
The inner layer of glass gained heat from the boiling water faster and expanded faster than the outer layer.
Step 3: Link to outcome
Hence, the uneven expansion of the glass surfaces caused the cup to crack.
You can download this Resource in PDF format to print or keep for future reference. Once you’ve submitted your request, it’ll be delivered straight to your email inbox.